lithium valence electron|What Are Valence Electrons? Definition and Periodic : Manila These elements, including hydrogen (H), lithium (Li), and sodium (Na), all have one electron in their outermost shells. That means that they can achieve a stable . Learn more about the full cast of Pitch Perfect 3 with news, photos, videos and more at TV Guide
PH0 · What Are Valence Electrons? Definition and Periodic
PH1 · Valency of Lithium
PH2 · Valences of the Chemical Elements
PH3 · Understanding the Lithium Molecular Orbital Diagram: A Complete Guide
PH4 · Understanding the Lithium Molecular Orbital Diagram: A
PH5 · Table of Oxidation States of the Elements
PH6 · Lithium (Li)
PH7 · Lithium
PH8 · How many valence electrons does Lithium (Li) have?
PH9 · How Many Valence Electrons Does Lithium (Li) Have?
PH10 · 3.7: Electrons and Valence Shells
PH11 · 3.10: Valence Electrons
crizen nicole / lyninii /ayesha sheenyberry scandal🔥 right away. Main channel @kfreemovie SPG Channel @viral_latest To AVAIL 👇🏼 PM Admin @FMovieAdmin2 Download
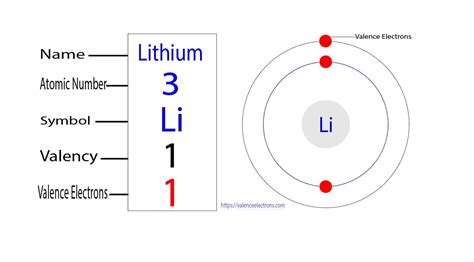
lithium valence electron*******Lithium has a single electron in the second principal energy level, and so we say that lithium has one valence electron. Li: 1s 2 2s 1 (the electron in the 2s energy level is the valence electron)These elements, including hydrogen (H), lithium (Li), and sodium (Na), all have .Element Lithium (Li), Group 1, Atomic Number 3, s-block, Mass 6.94. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images.
These elements, including hydrogen (H), lithium (Li), and sodium (Na), all have one electron in their outermost shells. That means that they can achieve a stable .

History. Lithium was discovered by Johann Arfvedson in 1817 when he was analyzing minerals from the island of Uto in Sweden. The pure metal was isolated the following year by both .lithium valence electron What Are Valence Electrons? Definition and Periodic For example, a lithium atom has 1 valence electron and has an oxidation state of +1. In contrast, a neon atom has 8 valence electrons and an oxidation state of 0.
Li+ valency is not zero like noble gas as their outermost shell has eight electrons. when a lithium atom loses one electron, Li+ ion is produced and that’s what valency is. May 19, 2024 May 25, 2014
lithium valence electronThe partially filled 2s orbital makes lithium a highly reactive element, as it readily donates its single valence electron to form stable compounds. This property of lithium also contributes to .
If you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
Lithium has a single electron in the second principal energy level and so we say that lithium has one valence electron. Beryllium has two valence electrons. How many valence electrons does boron have? You must recognize that the . The next atom, lithium, has an electron configuration of 1s 2 2s 1, so it has only one electron in its valence shell. Its electron dot diagram resembles that of hydrogen, except the symbol for lithium is used: \[\mathbf{Li}\mathbf{\cdot}\nonumber \] Beryllium has two valence electrons in its 2s shell, so its electron dot diagram is like that of . Thus, lithium has only one valence electron. Valency of Lithium (Li) There are many different ways to find out the valency of an atom which reflects the ability of an atom to bond with other atoms. Valence describes how easily an atom or a free radical can combine with other chemical species. The valency of an atom is determined based on the .
Here, we have mentioned the Lewis structure of the lithium. The lithium valence electrons dot diagram is as shown here: lithium – 1 s 2 2 s 1 – 1 valence electron. What is this electrons dot diagram is made up of? Electrons is the atom’s outmost energy level are the elections that are important in chemical bond and reactions. And these .Assigning Electron Configuration . We write electronic configurations by following the aufbau principle (from German, meaning “building up”). First we determine the number of electrons in the atom; then we add electrons one at a time to the lowest-energy orbital available without violating the Pauli Exclusion Principle .That is, recognizing that each orbital can hold two electrons, one .
Lithium has a single electron in the second principal energy level and so we say that lithium has one valence electron. Beryllium has two valence electrons. How many valence electrons does boron have? You must recognize that the second principal energy level consists of both the \(2s\) and the \(2p\) sublevels and so the answer is three.If you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.What Are Valence Electrons? Definition and Periodic Element Lithium (Li), Group 1, Atomic Number 3, s-block, Mass 6.94. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. . Members of a group typically have similar properties and electron configurations in their outer shell. Period A horizontal row in the periodic table. The atomic number of each element . Lithium has a single electron in the second principal energy level, and so we say that lithium has one valence electron. Beryllium has two valence electrons. How many valence electrons does boron have? Recognize that the second principal energy level consists of both the \(2s\) and the \(2p\) sublevels, and so the answer is three.Periodic Trends in the Oxidation States of Elements 1. Variation Of Oxidation State Along a Period. While moving left to right across a period, the number of valence electrons of elements increases and varies between 1 to 8. But the .The next element is lithium, with Z = 3 and three electrons in the neutral atom. . Definition of Valence Electrons: Definition of Valence Electrons, YouTube(opens in new window) [youtu.be] The general order in which orbitals are filled is depicted in Figure \(\PageIndex{2}\). Lithium has a single electron in the second principal energy level, and so we say that lithium has one valence electron. Beryllium has two valence electrons. How many valence electrons does boron have? Recognize that the second principal energy level consists of both the \(2s\) and the \(2p\) sublevels, and so the answer is three. Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical bonds. The number of valence electrons in one atom of each element is easily determined based on its position in the periodic table. For the main group elements (groups designated with a .
There are two ways to find the number of valence electrons in Lithium (H). The first is to use the Periodic Table to figure out how many electrons Lithium ha.Lithium's lower reactivity is due to the proximity of its valence electron to its nucleus (the remaining two electrons are in the 1s orbital, much lower in energy, and do not participate in chemical bonds). [10] . As an exception to the duet rule, a two-coordinate lithate complex with four electrons around lithium, [Li .The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus. Therefore, the number of electrons in neutral atom of Lithium is 3. Each electron is influenced by the electric fields produced by the positive nuclear charge and the other (Z – 1) negative electrons in the atom.
The next atom, lithium, has an electron configuration of 1s 2 2s 1, so it has only one electron in its valence shell. Its electron dot diagram resembles that of hydrogen, except the symbol for lithium is used: \[\mathbf{Li}\mathbf{\cdot} \nonumber \] Beryllium has two valence electrons in its 2s shell, so its electron dot diagram is like that .
Mailing Address. DLIFLC Office of the Registrar 597 Lawton Road, Bldg. 634, Rm. 4 Presidio of Monterey, CA 93944
lithium valence electron|What Are Valence Electrons? Definition and Periodic